What Is An Energy Profile Diagram
What is an energy profile. On an energy profile the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products.
 Electrophilic Aromatic Substitution Energy Diagram Organic Chemistry Chemistry Organic Chemistry Notes
Electrophilic Aromatic Substitution Energy Diagram Organic Chemistry Chemistry Organic Chemistry Notes
If you have done any work involving activation energy or catalysis you will have come across diagrams like this.

What is an energy profile diagram. This energy is given the symbol H and is different for different substances. An enthalpy diagram plots information about a chemical reaction such as the starting energy level how much energy needs to be added to activate. Energy profile diagrams for endothermic and exothermic reactions Every chemical substance has a certain amount of chemical energy.
Click on the following links to see earlier parts. The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram or sometimes called a reaction progress curve. An energy level diagram shows whether a reaction is exothermic or.
The following diagram illustrates the change in potential energy that occurs with rotation about the C2C3 bond. It shows the energy in the reactants and products and the difference in energy between them. It also shows the effect of a catalyst on the f.
Energy changes during a reaction can be displayed in an energy profile diagram. Sometimes reactions are more complex than simply a transition state Graph 3 which would represent a single step in the reaction mechanism. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions.
This diagram shows that overall the reaction is exothermic. For example the Activation Energy for the forward reaction. Enthalpy change H is the amount of energy absorbed or released by a chemical reaction.
This is part 3 of a four part series in the Energy Diagram Module. The model on the right is shown in conformation D and by clicking on any of the colored data points on the potential energy curve it will change to the conformer corresponding to that pointThe full rotation will be displayed by turning the animation on. Exothermic reactions When the activation energy is less than the energy released when the new bonds form there is an overall release of energy usually as heat released to the surroundings H is negative and an exothermic reaction has taken place.
Which of the following phase changes would this type of energy diagram refer to. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The minimum amount of energy needed by particles when they collide in order to cause a reaction is called the activation energy.
Now we must consider the activation energy the energy needed so that the reactants bonds will break and reform to make product Review of Endothermic Reactants Ep is lower than Products Ep. The products have a lower energy than the reactants and so energy is released when the reaction happens. Energy level diagram for an exothermic reaction is shown below.
The Activation Energy is the amount of energy needed to reach the top of the hill or Activated Complex. An energy level diagram shows whether a reaction is exothermic or endothermic. Going from the reactants to the top of the curve we are going up the energy axis of the graph.
Need to add more energy to the system for the forward reaction to take place. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Kay Review of Exothermic Reactants Ep is higher than Products Ep.
Once the reaction has obtained this amount of energy it must continue on. Stay tuned for Part 4. It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be easily measured.
Uncatalyzed reaction has a higher activation energy because there is no enzyme present in the reaction. The Activated Complex is an unstable intermediate product that is formed during the reaction. This may include primary energy used as raw fuels to feed into a system energy supply conversion or transformation losses and energy being used.
Energy Diagrams for Catalyzed and Uncatalyzed Reactions. This effect can be illustrated with an energy profile diagramCatalyzed reaction has a lower activation energy because there is an enzyme present in the reaction. The figure below shows basic potential energy diagrams for an endothermic A and an.
Potential Energy Diagrams Chemical Kinetics Mrs. Energy Flow Diagrams often also referred to as Energy Flow Charts are used to show energy and energy transformation visually and quantitatively.
 Energy Diagrams Sn1 And Sn2 Chemistry Help Potential Energy Organic Chemistry
Energy Diagrams Sn1 And Sn2 Chemistry Help Potential Energy Organic Chemistry
 Reaction Profiles Potential Energy Chemistry Profile
Reaction Profiles Potential Energy Chemistry Profile
 Chemistry 30 Chemical Kinetics Potential Energy Diagrams Revisited Chemistry Education Teaching Chemistry Chemistry
Chemistry 30 Chemical Kinetics Potential Energy Diagrams Revisited Chemistry Education Teaching Chemistry Chemistry
 Endo And Exo Diels Alder Energy Diagram Endo Chemistry Organic Chemistry
Endo And Exo Diels Alder Energy Diagram Endo Chemistry Organic Chemistry
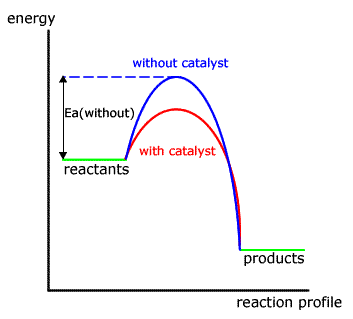 Energy Profile Diagram Google Sogning Organic Chemistry Chemical Reactions Chemistry
Energy Profile Diagram Google Sogning Organic Chemistry Chemical Reactions Chemistry
 Organic Chemistry Energy Diagrams Organic Chemistry Chemistry Physical Chemistry
Organic Chemistry Energy Diagrams Organic Chemistry Chemistry Physical Chemistry
 A Generalised Energy Profile Diagram For Kinetic Versus Thermodynamic Product Reaction Organic Chemistry Chemistry Physical Chemistry
A Generalised Energy Profile Diagram For Kinetic Versus Thermodynamic Product Reaction Organic Chemistry Chemistry Physical Chemistry
 Energy Profile Chemistry Chemistry Energy Organic Chemistry
Energy Profile Chemistry Chemistry Energy Organic Chemistry
 Energy Profile Diagrams Endothermic Exothermic Reactions 1 Exothermic Reaction Chemical Energy Energy
Energy Profile Diagrams Endothermic Exothermic Reactions 1 Exothermic Reaction Chemical Energy Energy
 Thesmartproject Endothermic Exothermic Reactions Energie
Thesmartproject Endothermic Exothermic Reactions Energie
 H Is A State Function Because E P V Are State Functions So It Depends Only On The Difference Betwee Teaching Chemistry Chemistry Education Science Chemistry
H Is A State Function Because E P V Are State Functions So It Depends Only On The Difference Betwee Teaching Chemistry Chemistry Education Science Chemistry
 Energy Profile Diagram Google Sogning Exothermic Reaction Energy Activities Classical Physics
Energy Profile Diagram Google Sogning Exothermic Reaction Energy Activities Classical Physics
 Activation Energy Chemistry Chemistry Experiments Energy Activities
Activation Energy Chemistry Chemistry Experiments Energy Activities
 E1 Energy Diagram Transition State Forming A Double Bond Energy Activities Reactions Chemistry
E1 Energy Diagram Transition State Forming A Double Bond Energy Activities Reactions Chemistry
 Energy Profile Of Catalyzed And Uncatalyzed Reactions Mcat Study Biochemistry Study Tools
Energy Profile Of Catalyzed And Uncatalyzed Reactions Mcat Study Biochemistry Study Tools
 Energy Profile Diagrams Endothermic Exothermic Reactions 3 Exothermic Reaction Chemical Energy Energy
Energy Profile Diagrams Endothermic Exothermic Reactions 3 Exothermic Reaction Chemical Energy Energy
 Sn1 Energy Diagram Transition States Activation Energy Intermediates Energy Activities Reactions Organic Chemistry
Sn1 Energy Diagram Transition States Activation Energy Intermediates Energy Activities Reactions Organic Chemistry
 Chemistry Rates Of Reaction Part 3 Exam Masters Tutoring Service Chemistry Energy Activities High School Chemistry
Chemistry Rates Of Reaction Part 3 Exam Masters Tutoring Service Chemistry Energy Activities High School Chemistry

Post a Comment for "What Is An Energy Profile Diagram"